
Saturday, April 29, 2006
What have cows got do with fairy rings?
 'May Day' stems from the fire festival of Beltane that celebrates the beginning of summer and the fertility of the coming year. Beltane is a Celtic word which means 'fires of Bel' (Bel was a Celtic deity). In springtime, at the beginning of the farming calendar, everybody would be hoping for a fruitful year for their families and fields. Most of the customs centre around flowers, greenery and at one time the 'hobby-horse'. So dancing round the maypole, dressing up as a fool with a bladder-on-a-stick and Morris dancing can all be blamed on Bel can it?
'May Day' stems from the fire festival of Beltane that celebrates the beginning of summer and the fertility of the coming year. Beltane is a Celtic word which means 'fires of Bel' (Bel was a Celtic deity). In springtime, at the beginning of the farming calendar, everybody would be hoping for a fruitful year for their families and fields. Most of the customs centre around flowers, greenery and at one time the 'hobby-horse'. So dancing round the maypole, dressing up as a fool with a bladder-on-a-stick and Morris dancing can all be blamed on Bel can it?It is said that a maiden can improve her looks by bathing her face in dew collected on a May morning. However, it’s not that simple. If the dew is collected from inside a fairy ring, then her appearance is turned into that of an old crone, complete with spots and blemishes. O-kaaaaaaay! I hear what you’re saying but come on! Is it really likely? Dew may be extremely pure water but if it had those kinds of properties don’t you think Estee Lauder might be onto it by now?
So what are fairy rings? Usually, a fairy ring is visible as a noticeable circle appearing in grass. Some rings look like a circle of grass that is darker at the edges than it is in the centre. Others look like a ring of poor-growth or bare earth. Fairy rings were thought to be the result of fairies dancing in a circle. No, don’t laugh! Another explanation held cow’s rear ends to blame! Cattle feeding on bales of hay invariably form a circle around their food, and what happens around the back? Yes, instant fertiliser, which means a circle of greener grass. Nice try, cow fans, but in actual fact fairy rings are all down to fungi.
The fairy ring was identified as a fungal growth pattern in 1792 by William Withering. It was due to a species called Marasmius oreades, whose modern common name is the fairy ring mushroom. This is one of 60 or so different species which produce fairy rings.
All fairy rings are produced in the same manner. Initially a spore (the fungal equivalent of a seed) lands on some suitable ground. It starts to grow underground, pushing out mycelium (fungal threads – the actual body of the fungus itself) in all directions. Eventually the central part dies off, leaving a disc of mycelium growing at the outer edge. Fungi release digestive chemicals (enzymes) into the ground to break down their food, which they then suck up. Unfortunately, they are sloppy eaters and not all of this digested food is taken up. The leftover nutrients are used by the surrounding plants. As a result of this, the grass grows more luxuriantly at the leading edge where the extra food supply is present. Meanwhile the death of the mycelium at the trailing (inner) edge returns nutrients to the soil, also stimulating a growth of grass.
Eventually fruit bodies (usually in the form of mushrooms) are produced to release spores and start the cycle again. Sometimes, several years may go by before mushrooms are actually seen at a fairy ring. Fairy rings may not be complete circles, as parts of the circle of mycelium become damaged and die off, leaving a crescent shape. So now you know! Fairies indeed…
Would dew believe it?
Would dew believe it? Finding the temperature at which the air begins to form dew is a way of gauging the air’s humidity.
You will need:
tin can
thermometer
crushed ice
bowl
water
What to do:
Half fill the bowl with crushed ice.
Ensure the outside of the can is completely dry.
Fill the can with cold water.
Place the thermometer in the can.
Add one tablespoon of crushed ice and stir.
Continue adding the ice until a layer of dew is visible on the outside of the can.
Immediately read the temperature on the thermometer to find the dew point temperature.
If it is high then the humidity is high also – watch out for the downpour!
What’s going on?
All air contains water vapour. As air cools (when it comes in contact with the cold can), the water vapour begins to condense. This is why glasses holding cold drinks "sweat" in the summertime. The dew point is the temperature at which moisture in the air begins to form dew. The higher the dew point temperature, the higher the moisture content of the air.
Read this for more information on humidity and the weather and for the full lowdown on weather click this.
You will need:
tin can
thermometer
crushed ice
bowl
water
What to do:
Half fill the bowl with crushed ice.
Ensure the outside of the can is completely dry.
Fill the can with cold water.
Place the thermometer in the can.
Add one tablespoon of crushed ice and stir.
Continue adding the ice until a layer of dew is visible on the outside of the can.
Immediately read the temperature on the thermometer to find the dew point temperature.
If it is high then the humidity is high also – watch out for the downpour!
What’s going on?
All air contains water vapour. As air cools (when it comes in contact with the cold can), the water vapour begins to condense. This is why glasses holding cold drinks "sweat" in the summertime. The dew point is the temperature at which moisture in the air begins to form dew. The higher the dew point temperature, the higher the moisture content of the air.
Read this for more information on humidity and the weather and for the full lowdown on weather click this.
Wednesday, April 26, 2006
You’re How Old?
 It might be rude to ask someone’s age, but they don’t have to tell you. Should they keep schtum, you can always estimate and you’d probably be correct plus or minus up to 10 years.
It might be rude to ask someone’s age, but they don’t have to tell you. Should they keep schtum, you can always estimate and you’d probably be correct plus or minus up to 10 years.Planet Earth has similarly tantalised scientists by refusing to directly reveal its age – but the history of our estimates of its age until recently produced figures that just kept on falling way, way short.
Earliest ideas about the age of the Earth were rooted in religious belief, and depending on your religion, could date the Earth from a few million years old to as young as a few thousand.
Geologists later tinkered with figures based on the number of layers of rock in a formation and an estimate of how long each layer took to be formed. These suggested a more ancient Earth than suggested by creation stories, but exactly just how ancient, they couldn’t say.
Then in 1862 the famous Lord Kelvin (a.k.a. William Thomson) weighed in, using ideas about heat transfer to estimate an age for Earth. He assumed it started off as a molten blob of rock and cooled steadily over the ages to its present temperature. He dated Earth to between 20 and 400 million years- an old Earth for sure, but an age that displeased biologists as it wasn’t a long enough timespan for natural selection to work its evolutionary magic. Like the biologists, geologists also reckoned this too young, but couldn’t say why or prove conclusively a more aged planet.
Another scientist (John Joly) came up with an estimate based on how salty the oceans were and how quickly erosion from the land dumped salt into rivers. Other scientists used astronomical techniques that also suggested an age like Joly’s of around 100 million years, so Kelvin’s figures looked reasonable in that light. But maybe they were too keen to side with the great man…
The discovery of radioactivity in 1896 killed off the idea of a ‘youthful’ 100 million year old Earth. This was a heat generating process that trashed assumptions made in Kelvin’s calculationsand wound the clock back to the presently accepted age of around 4.6 billion years …
For more info on how the Earth’s age is determined nowadays check out this site.
The Naked Egg
Hide your eyes! The egg is naked. Yes really. Have a go and you’ll see for yourself. Talk about setting a bad egg-zample…
You will need:
2 eggs
500 mL (2 cupfuls) vinegar (and some extra… just in case)
1 clear jar (clean jam jars work) or glass
Clock or timer
What to do:
Being careful not to crack the eggs, carefully place them in the jar or glass.
Pour enough vinegar over the eggs until they are completely covered (if 500 mL of vinegar is not enough, add more until covered).
Watch the eggs for about five minutes. Observe the bubbles of gas that are formed on the surface of the eggs; you'll notice that lots more will appear with time.
Let them sit overnight.
The next day, remove the eggs from the jar or glass and rinse them under a trickle of water in the sink while gently rubbing the shell with your fingers. If the shell does not come off completely, return the eggs to the jar or glass, and try again to rinse them the next day. It may take two or three days to remove the shell completely.
Once the shell is gone, examine the eggs carefully. Hold the eggs up to a bright window or light. You will see the yolk as a dark blob inside. Turn the egg upside down. Can you see the yolk "sinking" to the bottom of the egg?
What’s happening?
Eggshells contain something called "calcium carbonate." This is what makes them hard. Vinegar is an acid known as acetic acid. When calcium carbonate (the shell) and acetic acid (the vinegar) combine, a chemical reaction takes place and carbon dioxide (a gas) is released. This is what the bubbles are made of. The chemical reaction keeps happening until all of the carbon in the shell is used up - this takes about a day. When you take the eggs out of the vinegar, they are soft because all of the carbon escaped out of the shell in those little bubbles. The egg still stays together and doesn't fall apart because it has an "invisible membrane on the surface of it which does not react with the vinegar.
Now you know how to remove the eggshell without breaking it!
For more egg-citing egg-speriments click here.
You will need:
2 eggs
500 mL (2 cupfuls) vinegar (and some extra… just in case)
1 clear jar (clean jam jars work) or glass
Clock or timer
What to do:
Being careful not to crack the eggs, carefully place them in the jar or glass.
Pour enough vinegar over the eggs until they are completely covered (if 500 mL of vinegar is not enough, add more until covered).
Watch the eggs for about five minutes. Observe the bubbles of gas that are formed on the surface of the eggs; you'll notice that lots more will appear with time.
Let them sit overnight.
The next day, remove the eggs from the jar or glass and rinse them under a trickle of water in the sink while gently rubbing the shell with your fingers. If the shell does not come off completely, return the eggs to the jar or glass, and try again to rinse them the next day. It may take two or three days to remove the shell completely.
Once the shell is gone, examine the eggs carefully. Hold the eggs up to a bright window or light. You will see the yolk as a dark blob inside. Turn the egg upside down. Can you see the yolk "sinking" to the bottom of the egg?
What’s happening?
Eggshells contain something called "calcium carbonate." This is what makes them hard. Vinegar is an acid known as acetic acid. When calcium carbonate (the shell) and acetic acid (the vinegar) combine, a chemical reaction takes place and carbon dioxide (a gas) is released. This is what the bubbles are made of. The chemical reaction keeps happening until all of the carbon in the shell is used up - this takes about a day. When you take the eggs out of the vinegar, they are soft because all of the carbon escaped out of the shell in those little bubbles. The egg still stays together and doesn't fall apart because it has an "invisible membrane on the surface of it which does not react with the vinegar.
Now you know how to remove the eggshell without breaking it!
For more egg-citing egg-speriments click here.
Saturday, April 22, 2006
A Hatful of Cards Trick
 You will need:
You will need:A pack of cards.
A top hat. If you don't have a top hat, find a friendly Victorian gentleman and ask if you can borrow his. If that doesn't work, try an inferior sort of hat or - at a pinch - a cardboard box or saucepan. Just so long as the opening is fairly close to the ground, and about 15-20 cm wide.
What to do:
Try to drop the cards from approximately waist height into the hat. Go on, have a go.
No luck? Try challenging somebody else to do it.
They're not managing, either, eh? Shame. OK, skip ahead to the explanation, then come back here and offer them some sort of bet. Maybe they'll tidy your bedroom if you can drop five cards in a row into the hat?
Then proceed to drop every card, one after the other, into the hat. Which you can do, now you know the knack.
Hurrah! You've won the bet, showed the blighter who's boss, and generally saved the day. Time for a swift round of croquet before listening to pater's tales of his expedition to discover the source of the Thames, then hot milk and early to bed, all the better for more adventures tomorrow! Hurrah!
What's going on:
Pretty much everybody will try to aim the cards, dropping them vertically. Unfortunately they'll tumble furiously, and only the very occasional card will hit the hat. They tumble because they fall quickly, and the flow of air around them becomes turbulent, buffeting them around.
The trick is to drop the cards so they fall slowly. Hold them flat, by the edges, and let them gently parachute down into the hat. One or two might miss, but with a little practice it can be remarkably reliable.
When they're falling flat, the cards present a larger face to the air. Hence, they fall more slowly, and slip smoothly through the air. They'll still start to tumble if they pick up too much speed, however - it's worth testing your cards first to spot how high a drop is required for that to happen.
Friday, April 7, 2006
Year 11 Co-Science Mind Maps
 Just got time to put this on before the holidays start. Mr Ross has been producing mind maps for some of the Co-Science topics (core material only). You can download the file by clicking here (2Mb pdf - right click, file save as). Save it to your desktop and refer to it during your revision. Remember there are now 200 multiple choice co-science questions for you to have a go at - follow the instructions on this link. Three out of every four questions will from a previous paper and your paper 1 is worth 30% of your total mark.
Just got time to put this on before the holidays start. Mr Ross has been producing mind maps for some of the Co-Science topics (core material only). You can download the file by clicking here (2Mb pdf - right click, file save as). Save it to your desktop and refer to it during your revision. Remember there are now 200 multiple choice co-science questions for you to have a go at - follow the instructions on this link. Three out of every four questions will from a previous paper and your paper 1 is worth 30% of your total mark.
Monday, April 3, 2006
Periodic Table Breakout
 It's been a while since I gave you a 'break' in your revision game. Here's one with a chemistry theme (well this is a Science website). This is a classic game in which you have to clear the blocks to reveal the periodic table. There are more games on this site including some impossible missions, hangman, rotate some molecules and some chemistry thrown in there too. Happy revising if you are taking exams, happy playing if not!
It's been a while since I gave you a 'break' in your revision game. Here's one with a chemistry theme (well this is a Science website). This is a classic game in which you have to clear the blocks to reveal the periodic table. There are more games on this site including some impossible missions, hangman, rotate some molecules and some chemistry thrown in there too. Happy revising if you are taking exams, happy playing if not!
Solar Eclipse Data and Video
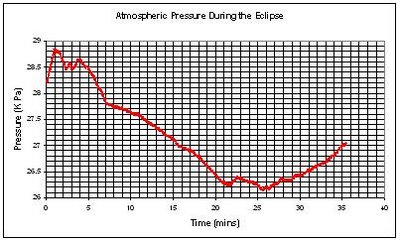
Take a look at the data sent to us from the British School of Lome in Togo, West Africa taken during the solar eclipse on Wednesday 29th April. There is also a video clip of the event that you can see on this link. See you in Indonesia on March 9th, 2016 for the next one in this part of the world. Thanks to Mr Jackson from the British School of Lome for sending us the data and video.



The Drinking Straw Propeller
 What you need:
What you need:Two drinking straws, bendy.
A pair of scissors, not bendy.
What to do:
Take one of the straws, and cut off the long end so you have about four centimetres of tubing on either side of the bendy bit.
Now bend the straw into a right-angle. Repeat the process with the other straw, but cut a slit in one end of it. Roll that end up, and slide it into the first straw to form a joint.
Arrange them so you have a 'U' shape lying flat on a table, then rotate the joint so one end points straight upwards. Take a look at the picture if that sounds confusing - it's really very easy.
Place one end of the straw contraption lightly in your mouth, and blow. With a little practice, it's possible to make the thing whizz around in a pleasingly silly manner, like some demented drinking straw propeller.
What's going on?
As the air blasts out it thrusts the straw around - action and reaction, Newton two, and all that. It's obvious, right?
Well, yes. But alas, it pains me to point out the complexity of the situation: When you suck, the propeller stubbornly refuses to spin the other way. Or, indeed, at all. Why?
Therein lies a tale, for this very problem has challenged some of the greatest minds of our time, most notably the Nobel Prize-winning physicist and bongo-player Richard Feynman. In such esteemed company I'm slightly nervous about giving a definite answer. Or, indeed, any at all. But we're all friends here so I'll wade in regardless.
Sucking on the straw is not the exact opposite of blowing down it. This is a common feature of aerodynamics - it's easy to blow out a candle, but sucking one out doesn't work nearly so well. And while you see many jet-propelled aircraft, I've yet to come across one that's powered by vacuum cleaners.
The difference is that when air is forced out of a nozzle, it's all travelling in roughly the same direction, so it comes out as a narrow jet. But when air is sucked into that same nozzle, it comes from all around the opening - not just directly in front, where the jet was, but from the sides, the top and bottom, and even behind the nozzle, along the tubing.
So while the jet is pushing against the air outside - and you can feel that reaction force as thrust - the sucked air is coming from all different directions, and there's no overall force.
Subscribe to:
Comments (Atom)



