Just one more to add to the list. I have never seen snow in Thailand (although it feels cold enough for some these days) but you can make you own with this great flash based site. Happy cutting!
Just one more to add to the list. I have never seen snow in Thailand (although it feels cold enough for some these days) but you can make you own with this great flash based site. Happy cutting!
Sunday, December 25, 2005
Make your own snowflake
 Just one more to add to the list. I have never seen snow in Thailand (although it feels cold enough for some these days) but you can make you own with this great flash based site. Happy cutting!
Just one more to add to the list. I have never seen snow in Thailand (although it feels cold enough for some these days) but you can make you own with this great flash based site. Happy cutting!
Levitation
You will need:
* A number of people (more than one for best effect)
* A narrow doorway
What to do:
Stand in the narrow doorway with your arms at your sides. Lift your arms up slightly so that you're touching the doorframe on either side with the backs of your hand
Now exert some effort! Press the back of each hand into the doorframe as hard as you can for 40 seconds.
Keep pushing…
Step away from the doorway and relax. What happens? Your arms will magically begin to levitate with no effort from you.
Get your group of friends to do the same thing and you can all stand around with your arms hanging in the air!
NB. The effect only lasts for a short time, so don't worry, you won't need to walk around like a penguin forever.
What's happening?
When you want a muscle to contract, your body sends an electrical signal along a nerve cell from the brain to the muscle. The signal starts a series of events that result in the contraction of that muscle.
The main event is the release of calcium ions into the muscle cells from the muscle's calcium store, or 'sarcoplasmic reticulum'. This increase in calcium makes the muscle contract.
When the nervous signal stops, the calcium ions go back into the sarcoplasmic reticulum and the muscle can relax.
To lift your arm from your side you use two muscles in your shoulder, the deltoid and supraspinatus muscles. By pressing your arm against the doorway you are sending a command to these muscles to move, but they can't because the doorframe's in the way.
As you carry on pushing against the doorway, you are still telling your muscles to move. A stream of electrical signals arrives at the nerve endings in these muscles. Masses of calcium ions are released into the muscle cells and they can't be completely cleared back into the sarcoplasmic reticulum.
When you move away from the wall, there is so much calcium in your muscles and they can finally contract in response! So even though the command from your nerves has stopped, your arm will lift itself away from your side!
* A number of people (more than one for best effect)
* A narrow doorway
What to do:
Stand in the narrow doorway with your arms at your sides. Lift your arms up slightly so that you're touching the doorframe on either side with the backs of your hand
Now exert some effort! Press the back of each hand into the doorframe as hard as you can for 40 seconds.
Keep pushing…
Step away from the doorway and relax. What happens? Your arms will magically begin to levitate with no effort from you.
Get your group of friends to do the same thing and you can all stand around with your arms hanging in the air!
NB. The effect only lasts for a short time, so don't worry, you won't need to walk around like a penguin forever.
What's happening?
When you want a muscle to contract, your body sends an electrical signal along a nerve cell from the brain to the muscle. The signal starts a series of events that result in the contraction of that muscle.
The main event is the release of calcium ions into the muscle cells from the muscle's calcium store, or 'sarcoplasmic reticulum'. This increase in calcium makes the muscle contract.
When the nervous signal stops, the calcium ions go back into the sarcoplasmic reticulum and the muscle can relax.
To lift your arm from your side you use two muscles in your shoulder, the deltoid and supraspinatus muscles. By pressing your arm against the doorway you are sending a command to these muscles to move, but they can't because the doorframe's in the way.
As you carry on pushing against the doorway, you are still telling your muscles to move. A stream of electrical signals arrives at the nerve endings in these muscles. Masses of calcium ions are released into the muscle cells and they can't be completely cleared back into the sarcoplasmic reticulum.
When you move away from the wall, there is so much calcium in your muscles and they can finally contract in response! So even though the command from your nerves has stopped, your arm will lift itself away from your side!
Bored?
 Ok, have you broken the new present Santa gave you already? You've watched all the new movies on UBC ages ago on DVD? Just waiting for the batteries on your PSP to recharge? Have a look at these two sites. You can create your own festive rap song (surprisingly addictive) or do a classic word search. Nothing to do with Science, but just something to kill the time and annoy your family with! Happy holidays.
Ok, have you broken the new present Santa gave you already? You've watched all the new movies on UBC ages ago on DVD? Just waiting for the batteries on your PSP to recharge? Have a look at these two sites. You can create your own festive rap song (surprisingly addictive) or do a classic word search. Nothing to do with Science, but just something to kill the time and annoy your family with! Happy holidays.
Friday, December 2, 2005
How fast are you?

Think you're fast? Try this clever little trick to work out how fast your reaction time is – and there is no need for high precision clocks!
You will need:
A ruler, or metre stick if you have one
Some volunteers who are prepared to be tested…
What to do:
Hold the ruler at one end, so that the zero is at the bottom, hanging down between your volunteer's thumb and forefinger.
Tell your volunteer that you will drop the ruler some time within the next 5 seconds and their job is to close their thumb and forefinger to catch it as fast as they can after it starts dropping.
Drop it…and record where they managed to catch it. Keep testing them and anyone else wants to try!
What's going on:
Here's a very rough guide to the reaction times taken to grab the ruler at different distances down the ruler…
5 cm = 0.10 seconds
10 cm = 0.14 seconds
15 cm = 0.17 seconds
20 cm = 0.20 seconds
25 cm = 0.23 seconds
30 cm = 0.25 seconds
43 cm = 0.30 seconds
60 cm = 0.35 seconds
80 cm = 0.40 seconds
This experiment measures how long it takes for visual information to reach the brain, and for the brain to process the information, and send a command to your hand to say 'grab NOW!!!'
Whatever time you recorded, you must admit it's pretty speedy considering everything that has to happen inside the brain - plus the time it takes the 'grab now' message to travel from Brain Central out to the Fingers Operational Depot!
You can even take the experiment further; try comparing boys and girls, people of different ages, and you can even turn the lights down…
It's a Cracker!

You will need:
A packet of crackers - dry and fairly big!
A jug of water
Some volunteers
A watch with a second hand
What to do:
Challenge your volunteers to eat three crackers in under a minute, with no water, butter or other lubricant.
Give them a drink of water when the minute is up!
If you volunteers go separately they can try different techniques like shoving the crackers all in at once or breaking them into small pieces first.
What’s going on?
It is almost impossible to eat three crackers in under a minute because our bodies do not manufacture enough saliva to get the crackers to go down. Saliva is a very clever substance; not only does it provide enzymes to start breaking down food in your mouth it is absolutely essential for lubricating the mouth and throat to assist with swallowing. It also helps us taste, and keeps down infections in the mouth.
Our bodies manufacture between 1 and 2 litres of saliva each day, most of which ends up in our stomachs and is digested again. Unfortunately the crackers are very dry, great for storage, but not so great if you are trying to eat loads at once.
If you want to delve a little deeper you can get one of your volunteers to keep a cracker in their mouth for a while, while the enzyme amylase in their saliva gets to work and turns the starch in their cracker to sugar. It should start to taste sweet.
Warning - don't try this alone and do have a drink of water ready as it can cause choking if you aren't careful!
What happens when Science meets Culture?

Click on the image above to launch the page. When loaded drag the dot in the bar at the bottom left and right to answer the question above. The images link to current Science articles. If you are impressed by this then explore more projects by Jonathan Harris here.
MP3's too late for the Yr11 Trial Examinations
 It has been some time since the last post. Perhaps time to push that horrible photo down out of view? I came across some excellent revision MP3's on the BBC bitesize website. The subjects include Physics, Chemistry, Biology and English. You can use the BBC website to download 'bitesize' chunks or use the Patana Science page and click on the podcasts icon on this page to download the whole lot in one go. Great for revising on the bus to school. By the way - does anybody listen to the podcasts I put there? Comments please.
It has been some time since the last post. Perhaps time to push that horrible photo down out of view? I came across some excellent revision MP3's on the BBC bitesize website. The subjects include Physics, Chemistry, Biology and English. You can use the BBC website to download 'bitesize' chunks or use the Patana Science page and click on the podcasts icon on this page to download the whole lot in one go. Great for revising on the bus to school. By the way - does anybody listen to the podcasts I put there? Comments please.
Sunday, November 20, 2005
Getting Sorted
 It’s one of those common questions – how does the science you do in the classroom work in real life? Here’s how density crops up every day, as we all try to be a little greener…
It’s one of those common questions – how does the science you do in the classroom work in real life? Here’s how density crops up every day, as we all try to be a little greener…You will need:
Different plastic cartons such a milk bottle, yoghurt pot, washing up liquid bottle
Scissors
A large bowl of water
A tablespoon
Salt
What to do:
Check the code on the bottom of your containers. There should be a number inside the triangular recycling symbol; for example a plastic milk bottle is code 2 or HDPE
Cut out three strips, about 1cm x 4cm, from each plastic container.
Fill the bowl with water and place the strips in it making sure they are fully immersed. Try adding washing up liquid if the strips cling to the surface.
One set of strips will float to the surface immediately. Remove these strips.
Now add a large tablespoon of salt to the bowl and stir it up so that it dissolves. You may need to keep adding more until another set of strips float to the surface. Remove these strips.
There should be one set of strips still sitting at the bottom of the bowl.
Congratulations! You have now successfully sorted three different plastics!
What’s going on?
Each plastic container is made of a different plastic, and has its own 'density'. Density is the measure of how heavy something is for its size, in other words its mass per unit volume.
Water has a density of 1 g/cm3, and anything with a density greater than this will sink in water; anything with a lower density will float. So if we have a plastic with a density less than 1 (e.g. the milk bottle) it will float in water.
Adding the salt to the water makes it more dense. That's why we float more easily in the sea than in a swimming pool. The yoghurt pot has a density slightly greater than 1 g/cm3, so it doesn't float in tap water but it will float in salt water. The washing up liquid bottle however has a higher density still, which means that it won't float in either tap or salt water.
This is how they sort plastic for recycling, which is why you can drop all sorts of different cartons into one enormous skip!
Monday, November 14, 2005
Funny but serious
 A couple of weeks ago Mr Moakes and I were showing the Year 7 students the dangers of smoking as part of their tutorial programme. Did you know you can find arsenic and polonium in cigarettes? This site tells you about all the 4000+ different chemicals in cigarettes. Click the image left to watch a very funny video (with a serious message) about the damages of smoking. (2Mb)
A couple of weeks ago Mr Moakes and I were showing the Year 7 students the dangers of smoking as part of their tutorial programme. Did you know you can find arsenic and polonium in cigarettes? This site tells you about all the 4000+ different chemicals in cigarettes. Click the image left to watch a very funny video (with a serious message) about the damages of smoking. (2Mb)
Sunday, November 13, 2005
What Happens When the Arctic Melts?
 We don’t often see frozen sea water, but, of course, that is what the north pole is! Ever wondered what would happen if the Arctic ice cap melts? Here is a clever demonstration which shows you just that!
We don’t often see frozen sea water, but, of course, that is what the north pole is! Ever wondered what would happen if the Arctic ice cap melts? Here is a clever demonstration which shows you just that!You will need:
A drinking glass
Ice
Marker pen
Ruler
What to do:
Fill a glass three-quarters full with water and add ice. Mark with your marker pen a line along the water level and measure the height from the bottom of the glass to the line, then wait for the ice to melt…
Once the ice melts, mark the new water level line and measure the new height from the bottom of the glass to the line. There should be a small drop in water level.
What’s happening?
Ice (unlike most other things) is actually bigger in its solid form than in its liquid form, and so it shrinks as it melts! The result of this experiment means the melting of sea ice has nothing to do with rising sea-levels - but global warming does lead to a rise in sea-level.
This is because even though water is unusual and expands when it freezes, it still expands when it warms up. The global rise in temperature has meant the water in our seas has got bigger, and so the overall global sea level has risen because of this.
Melting of land ice such as in the Antarctic also leads to a rise in sea-level!
Thursday, November 10, 2005
Magic or Science?
 With chemistry week sadly fizzling to an end, I came across this site all about the 'darker' side of Sir Isaac Newton. A brilliant mathematician and physicist, did you know he was also into alchemy? In fact during this period in history he was not the only scientist in pursuit of making gold from other chemicals, Robert Boyle, John Locke, Leibniz and many others were trying. Read an interview with Historian Bill Newton on Newton's alchemy on this link. Also have a look at this nice flash site that decodes Newton's notebooks. I wish I had a similar tool for Zak's physics homework.
With chemistry week sadly fizzling to an end, I came across this site all about the 'darker' side of Sir Isaac Newton. A brilliant mathematician and physicist, did you know he was also into alchemy? In fact during this period in history he was not the only scientist in pursuit of making gold from other chemicals, Robert Boyle, John Locke, Leibniz and many others were trying. Read an interview with Historian Bill Newton on Newton's alchemy on this link. Also have a look at this nice flash site that decodes Newton's notebooks. I wish I had a similar tool for Zak's physics homework.
Sunday, November 6, 2005
Fire Extinguisher!
 Activity: Putting Out the Light
Activity: Putting Out the LightIf you are off to enjoy the fireworks displays this weekend, you might feel inspired to try some fire activities of your own, or perhaps you are wondering about the best way to put the flames out!
You will need:
Small candle (like a nightlight) and matches
Vinegar, hot water (from the hot tap) and bicarbonate of soda
A jug with a lip that you can pour from
Another container to mix the water and vinegar
What to do:
In your container, prepare a hot water and vinegar mix, (50-50 of each).
Light the candle.
Put about a dessertspoon of baking powder in your jug, then pour on the vinegar-water mix.
Let the fizz subside for a second or so, then take the jug and carefully ‘pour’ the carbon dioxide produced in the fizzing, over the candle. It will go out, ‘suffocated’ by the lack of oxygen.
What’s going on?
A candle burns in oxygen, combining oxygen with the chemicals that make up the candle wax creating water, carbon dioxide and a bunch of other bits and pieces. If you remove the oxygen, or displace it, the candle will go out.
The mix of baking powder and vinegar produces carbon dioxide gas in the fizzing that you see. Carbon dioxide is heavier than air so when you lean the jug over the candle carbon dioxide pours out and sinks onto the flame extinguishing it.
This experiment comes from Planet Science’s Little Book of Experiments – have a look here for more science things to do with candles and other stuff you’ll find in your home.
You could be a winner!
 To celebrate Chemistry Week and all things elemental there is an elementary quiz on this link - enter and you have the chance to win a one of three ‘Tungsten’ T’s, RSC goodies and copies of Bill Bryson’s ‘A Short History of Nearly Everything’. Remember the clues are in the questions! On the chemistry theme (and of course November 5th Guy Fawkes/Bonfire Night) - take a look at this fireworks website - it is one of the best I have seen - lots of flash animations on how they work and videos of the different types, you can even play around in their virtual fire lab, rearrange some molecules and find out the temperature of a striking match. The practical of the week is interestingly all about putting fires out quickly!
To celebrate Chemistry Week and all things elemental there is an elementary quiz on this link - enter and you have the chance to win a one of three ‘Tungsten’ T’s, RSC goodies and copies of Bill Bryson’s ‘A Short History of Nearly Everything’. Remember the clues are in the questions! On the chemistry theme (and of course November 5th Guy Fawkes/Bonfire Night) - take a look at this fireworks website - it is one of the best I have seen - lots of flash animations on how they work and videos of the different types, you can even play around in their virtual fire lab, rearrange some molecules and find out the temperature of a striking match. The practical of the week is interestingly all about putting fires out quickly!
Wednesday, November 2, 2005
Nothing to do with Science
Here is food for thought, type the word 'failure' into the google search box to the right and see the top link!
Monday, October 31, 2005
Chemistry Week
 The Royal Society of Chemistry are promoting chemistry for one week from Friday 4th November. In support of this there are some interesting articles on the following topics:
The Royal Society of Chemistry are promoting chemistry for one week from Friday 4th November. In support of this there are some interesting articles on the following topics:Click on the links to see what all of them have to do with chemistry - you'll be surprised! If you are really bored there is a tile sliding game at this link! Happy sliding :)
An Excellent Book
 This really is a good read that I would like to recommend.
This really is a good read that I would like to recommend.It's written by three English children whose mother takes them from England to live in Botswanna. There they enjoy many adventures and play an important role in the study of Lions. 'Better than any novel' says the Guardian Newspaper.
ISBN 184255 220 1
Friday, October 28, 2005
Seeing Ghosts!
 You will need:
You will need:2 sheets of white paper
A red felt tip pen
What to do:
Draw a ghost in red on one of the sheets of paper and colour it in.
Stare at it for about 30 seconds… you’ll start to see a green outline around it when your eyes are ready.
Now look at the second, blank sheet of paper.
You should see a ghostly green image hovering on the paper!
What’s going on?
Eyes get tired like any other part of the body. When you look at a red image the cells in your eyes’ retinas respond to the colour, but after a while they get tired and stop functioning as well. White light is made up of all colours, so when you then look at a white sheet of paper not all the red light is picked up by your tired eyes and you are left with an after image of green.
Click here to download some ghost drawings you can colour in & trace.
Saturday, October 22, 2005
An Edible Solar System
 Here’s one we made earlier… but if you missed it here is your chance to get all space age in the kitchen. What could be nicer now it is getting wintry than a hot chocolate and a solar system biscuit!
Here’s one we made earlier… but if you missed it here is your chance to get all space age in the kitchen. What could be nicer now it is getting wintry than a hot chocolate and a solar system biscuit!You will need:
- 175g plain flour
- 100g butter or margarine
- 50g caster sugar
- Four different sized biscuit cutters
- Items to decorate – coloured icing, hundreds and thousands and liquorice
- Pre-heat the oven to 150°C/300°F Gas 2 (get an adult to do this!)
- Cream the butter or margarine and caster sugar together until they are light and fluffy.
- Stir in the flour and, once mixed, knead the dough together until it forms a ball.
- Add a sprinkle of flour if the dough is sticky.
- Roll out the dough on a lightly floured surface until it is about 5mm thick.
- Use the smallest biscuit cutter to cut three biscuits from the dough (Pluto, Mercury and Mars).
- Use the next-size-up biscuit cutter to make two biscuits (Venus and Earth).
- Use the next larger biscuit cutter to make another two biscuits (Neptune and Uranus).
- Use the largest biscuit cutter to cut the last two biscuits (Saturn and Jupiter).
- Place the biscuits on a baking tray and bake in the centre of the oven for 25 minutes or until golden brown.
- Let the biscuits cool before decorating.
- Keep track of the planets as you cut them out so you can decorate them correctly after they are cooked.
Now for the decoration:
- Mercury has a rocky surface and is orange-red in colour, so use coloured icing and hundreds and thousands to decorate this biscuit.
- Venus is covered with thick, yellow clouds so you will need yellow icing.
- Earth is an obvious one! Decorate with green and blue icing and a sprinkle of icing sugar to resemble the clouds.
- Decorate your Mars biscuit with red icing.
- Jupiter is a giant ball of yellow, orange and red gas arranged in stripes.
- Use stripes of coloured icing decorate with a red sweet in the middle to resemble Jupiter’s Great Red Spot.
- Saturn looks yellow because of its foggy atmosphere and is famous for its rings, so use yellow icing and lay a few pieces of liquorice on the biscuit to resemble its rings.
- Uranus looks green so decorate with green icing.
- Neptune is blue with faint stripes so decorate with blue icing and make faint stripes with sprinkles of icing sugar.
- Finally for Pluto, sprinkle a little icing sugar on the top of the biscuit to resemble this icy, rocky planet.
Now all you have to do is arrange the biscuits in the correct planetary order, and serve!
Friday, October 21, 2005
An important message from 8V!
Neon, Vanadium, Erbium, Ruthenium, Nitrogen, Indium, Lanthanum, Boron, Sulphur. A very important message they pose. If you think you know the answer - ask someone in 8V that knows!
Wednesday, October 19, 2005
Guest speaker Dr Azeez Abdul Hakeem
 If you were not lucky enough to see and hear the talk given to us by Dr Azeez Abdul Hakeem on the environmental projects at the Banyon Tree in the Maldives then don't worry, you can hear his talk by clicking on the podcasts icon or simply click here. Many thanks for Dr Azeez for his time and to Miss Brown for organising the event.
If you were not lucky enough to see and hear the talk given to us by Dr Azeez Abdul Hakeem on the environmental projects at the Banyon Tree in the Maldives then don't worry, you can hear his talk by clicking on the podcasts icon or simply click here. Many thanks for Dr Azeez for his time and to Miss Brown for organising the event.
Monday, October 17, 2005
Be a young Galileo
 Activity: Galileo and his Pendulums
Activity: Galileo and his PendulumsOne of the things that Galileo was most famous for was pointing out that something’s mass does not affect how quickly it falls to the ground. Tricky to see when there is air resistance and a lack of high places that are safe to drop heavy weights from!
You will need:
String
Modelling clay or plasticene
Weighing scales
What to do:
Take the modelling clay and make two pendulum bobs of different weights. Make one considerably heavier than the other, but not so great that the sizes are vastly different. Keep them spherical
Attach them each of them to piece of string of same length to make two pendulums.
Stick them in an open doorway or along a support, again make sure the string length is the same.
Pull them out to the same height at the same time and let go…
They should be swinging perfectly in unison, if you have made them carefully enough!
What’s going on?
Pendulums don’t just swing, the weights at the end are actually falling. The pendulum motion allows them to ‘fall’ for longer than they would if we chucked them out a window! Which means we can examine how different masses are affected by gravity.
When compared to a falling hammer or feather we might expect the lighter weight to fall more slowly than the heavier one. But if you watch your pendulums you’ll see this is not the case. It doesn’t matter what the mass is, all objects fall at the same rate, as long as you rule out air resistance. It is tricky to get rid of air resistance but you can minimise the effect by making the bobs similar sizes and spherical!
Sunday, October 16, 2005
Do girls really have the X factor?
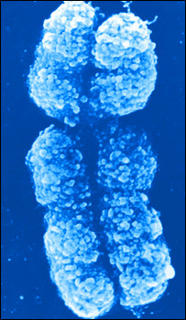 Have you been watching the X factor? Are the girls doing better than the boys? Well they should do because girls have double the X factor. That is to say female mammals have two X chromosomes whereas males have one X and one Y. What’s more, the X chromosome contains a thousand more genes than the Y. Hold on girls! Before you start your goal celebration routines it seems the female body switches off one X chromosome - quite randomly - in each cell, thus evening up protein production between the sexes. Our one chance to be the superior sex and nature decides to intervene!
Have you been watching the X factor? Are the girls doing better than the boys? Well they should do because girls have double the X factor. That is to say female mammals have two X chromosomes whereas males have one X and one Y. What’s more, the X chromosome contains a thousand more genes than the Y. Hold on girls! Before you start your goal celebration routines it seems the female body switches off one X chromosome - quite randomly - in each cell, thus evening up protein production between the sexes. Our one chance to be the superior sex and nature decides to intervene!So what does all this mean? Well did you realise that tortoiseshell cats - which are nearly always female - have a gene for one colour on one of their X chromosomes and a gene for another colour on the other X chromosome? This leads to the multicoloured patterning.
Also females are protected from many diseases because of their double dose of the X chromosome. A host of nasty diseases and disorders sit on the human X chromosome, including haemophilia. But because females have another - usually healthy - copy of the X chromosome, they are usually shielded from the full impact of these disorders. Males, on the other hand, have nothing to fall back on.
But wait for this - although the average IQ of men and women is equal, men are more frequently found at both extremes of intelligence. In a nutshell, you’ve either got it – or you haven’t! This is because, if you have very good intelligence genes on your X chromosome, it pays not to have them muffled by more average genes on another X chromosome.
For more details click here.
Monday, October 10, 2005
Tuesday, October 4, 2005
Levitating paperclips

How does the magnet attract the paperclip?
What is the resultant force?
Is the system in equilibrium?
What is lightning?
 Did you sleep through the electrical storm last night? There were so many lightning strikes I simply pointed my digital camera out the window and took a few snaps (see image). Do you know what causes lightning? Have you ever heard of ball lightning? Do you know the safest place to be when it strikes? Some facts about lightning:
Did you sleep through the electrical storm last night? There were so many lightning strikes I simply pointed my digital camera out the window and took a few snaps (see image). Do you know what causes lightning? Have you ever heard of ball lightning? Do you know the safest place to be when it strikes? Some facts about lightning: - A lightning charge contains 30 million volts at 100,000 amperes.
- The total energy in a large thunderstorm is more than that in an atomic bomb.
Use the links below to read more about this natural phenomenon.
http://www.livescience.com/forcesofnature/lightning_science.html
(how lightning forms)
http://www.abc.net.au/science/news/stories/s520317.htm
(how ball lightning forms)
http://www.lightningsafety.com/nlsi_pls/lst.html
(how to avoid getting hit by lightning)
Thursday, September 29, 2005
A tricky question
 Now here's a little quiz in which even the biology teachers can participate. We know that the sperm cell only delivers its nuclei to the egg cell at fertilisation. Why is it that the 2 cells (egg and sperm)do not simply fuse all of their cytoplasm together? It is known that none of the organelles of the sperm cell are delivered to the egg, only the nuclei.
Now here's a little quiz in which even the biology teachers can participate. We know that the sperm cell only delivers its nuclei to the egg cell at fertilisation. Why is it that the 2 cells (egg and sperm)do not simply fuse all of their cytoplasm together? It is known that none of the organelles of the sperm cell are delivered to the egg, only the nuclei.So, why?
Monday, September 26, 2005
States of Matter

7K investigate states of matter.
The copper melts and falls into the beaker of water. What happens as the melted liquid coper hits the water?
Thursday, September 22, 2005
Thursday, September 15, 2005
Who is the owner of this shoe?
 Over the holiday the science office moved around the corner to accomodate the new labs. All the 'junk' was packed in boxes and it is only until now that I have started to open some of them. In one I found this gem that I have to share with you. Last year Mr Lazaro asked his yr 10 chemistry class to do a homework on the uses of zinc. I remember seeing a quality flash animation and some interesting models, but not this. The cd was tucked away in the bottom of a box with no label so as I put it into the cd drive I was expecting to see some boring past papers! To my surprise I heard this on the cd (as a podcast). Even better, you can click here to see what was on the cd (if you are in school) or here if you are at home (47 MB mpeg). Sorry Paulo.
Over the holiday the science office moved around the corner to accomodate the new labs. All the 'junk' was packed in boxes and it is only until now that I have started to open some of them. In one I found this gem that I have to share with you. Last year Mr Lazaro asked his yr 10 chemistry class to do a homework on the uses of zinc. I remember seeing a quality flash animation and some interesting models, but not this. The cd was tucked away in the bottom of a box with no label so as I put it into the cd drive I was expecting to see some boring past papers! To my surprise I heard this on the cd (as a podcast). Even better, you can click here to see what was on the cd (if you are in school) or here if you are at home (47 MB mpeg). Sorry Paulo.
Tuesday, September 13, 2005
No bags please
 The science corridor is a 'bag free zone'. Please leave your bags in your lockers.
The science corridor is a 'bag free zone'. Please leave your bags in your lockers.If we need to evacuate the laboratories for any reason we need the floors clear of obstacles.
This is a school rule and you older students should know better.
Thursday, September 8, 2005
Not another way to learn!!!!!!!!!!!!!

Listen and Learn... are you always able to remember a tune, song or lyrics, but struggle to recall facts in your exams? Many students who have a strong auditory memory find information sticks if they hear it rather than read it. At Patana the Science department will be putting together a list of mp3's that you can download and listen to whenever you like, on the school bus for example! Click here to go to a selection of mp3's that you can download now. Bookmark the page and visit it on a regular basis since new files will be added every week. Ever tried to play tennis on the moon? What would happen to Andy Roddick's 150 mph serve on the moon?Happy listening.
Monday, September 5, 2005
7R's Lab Rules
 We always have to be safe in the Science Laboratories. 7R have come up with the following rules to help us stay safe, can you add to them?
We always have to be safe in the Science Laboratories. 7R have come up with the following rules to help us stay safe, can you add to them?1. Never run in labs.
2. Always wear lab coats when experimenting.
3. Leave bags outside the lab.
4. Tuck stools under the desks when experimenting.
5. Keep surfaces clear when experimenting.
6. Always light Bunsen burners on a safety flame.
Tuesday, August 30, 2005
Building the new labs

Do you remember what labs 9 and 10 used to look like? We have three new labs that were completed (just in time) over the summer. There are plans to make the others have a similar format next summer holiday. Here is a photo of what the prep room looked like before it was turned into corridor! The lab technicians are working very hard to sort out all the equipment and pack it back into the two new prep rooms. Our technicians are called Khun Gun (pronounced Gan), Khun Ohm and Khun Pornpen. Next time you see them in the corridor or classroom say hello, ask them if they need any help! Sometimes we forget how much work goes on behind the scenes so that you can do practicals in your lessons.
Monday, August 29, 2005
Tuesday, July 5, 2005
Barbie Bungee Challenge

Thanks to all those involved in the Year 10 Barbie Bungee Challenge. The judges are still deliberating over the winning presentation. You can see the video of all the jumps and interviews with the designers by clicking this link when in school only (the 100MB *.mpeg file is in student shared>year 10>science). Enjoy the holidays.
Tuesday, June 28, 2005
Electrolysing Red Cabbage!

Ok, we've all made an indicator out of red cabbage, it's in Topic 6 of Spotlight 7. But can you explain how a solution of "red cabbage dye" shows these colours when a current is passed through it?
Clue - salt was added to the solution...but what was this for? Ask a Chemistry Clubber for more help!
Friday, June 24, 2005
Did you see the halo in the sky today?

Today some of the students saw a halo around the sun. Check out this website to find out more about halos, auroras and the mysterious phenomena called the Specter of the Brocken.
Thursday, June 23, 2005
How good was your revision?

Now that your Annual exams are over take some time to reflect on how good your revision was. What techniques worked best for you? Flash cards? Mnemonics? Recording on to your mp3 player? There are many ways to revise and all of us remember things in different ways. Try some of the ideas on this "buzzin" web site. There are many more sites at the bottom of the KS4 past papers page that are good for all ages. Many of your teachers mentioned that you were not reading the question carefully. Here is a helpful list (with definitions) of the terms that are used by the examiners in Science exams. Print it off and stick it on the inside cover of your book or folder so that you see it every day. Highlight these words in your paper so that you know what the examiner requires in the answer.
Tuesday, June 14, 2005
Invigilating your exams

It has been a long time since the last post. I apologise. Perhaps the boredom of invigilating your Annual Exams has set in. Or is it the prospect of marking all those scripts in the next couple of weeks? I am sure you are not so sympathetic to us doing all that marking. May times I hear "Don't set such long papers then!". I took this photo today with a mobile phone (hence the low resolution) because there were not many lessons going on in the department at the time. Don't tell Mr Platt - I was supposed to be watching for all those cheat notes and equations written on the lids of your calculators! On a more serious note, Mr Burrell and I were thinking about giving you more control and ownership of the look of the Science site and came up with a couple of ideas. The current home page could be split into KS3 (years 7-9), KS4 (years 10-11) and KS5 (years 11-12). In other words a separate page or blog for each. Interested students from each KS could work on the design, add their own posts/pictures/ideas etc whilst keeping the menu structure (link and learn, past papers, revision notes etc) on each of the pages. The question is, who is going to do it? Well, that's really up to you. If you answer yes to any of the following questions then maybe you would like to get involved. Are you interested in Science? Do you like using computers and the internet? Bored at lunchtime? Need some hours for your CAS? Your cv is not too hot or your UCAS form needs spicing up? Anyone from any year group can take part - just post a comment with your first name and tutor group or email us (see the link under hot links) and we will get back to you. Mr Burrell runs a 'How to write your own blog' ECA two afternoon's per week and perhaps this could be part of it. Why not get involved? Ok, I'll shut up now :)
Monday, June 13, 2005
Thursday, June 9, 2005
Do you want to improve your exam grade?
Read these two articles, one on how not to waste marks, and the other on how to interpret a question properly, from Catalyst magazine. The full set of Catalyst magazines is available in the Resources Centre.
Monday, June 6, 2005
One snack card gone, two more left!
 Mr Moore's challenge certainly got some of you mythical creatures thinking hard. We have a winner with Fah in 8S. Her order of Griffin > Kinnaree > Satyr > Chimaera > Echidna > Sphynx > Pegasus > Centaur > Minotaur won the tastefully framed snack card you see left. But wait, there are two more cards to anyone handing in, to the Science Office or SDRC Issue Desk, a set of pictures that correctly line up the creatures (but you can't use Fah's sequence). What could be more tempting than making Mr Moore give up his precious (aka Gollum) snack cards!
Mr Moore's challenge certainly got some of you mythical creatures thinking hard. We have a winner with Fah in 8S. Her order of Griffin > Kinnaree > Satyr > Chimaera > Echidna > Sphynx > Pegasus > Centaur > Minotaur won the tastefully framed snack card you see left. But wait, there are two more cards to anyone handing in, to the Science Office or SDRC Issue Desk, a set of pictures that correctly line up the creatures (but you can't use Fah's sequence). What could be more tempting than making Mr Moore give up his precious (aka Gollum) snack cards!
Friday, June 3, 2005
Miss Sackey needs your help
If you have a few minutes Miss Sackey would be grateful if you could answer some questions about the experiments we do in your Science lessons and the way that you learn. Click here to go to the questions.
Wednesday, June 1, 2005
Revision, where to start?
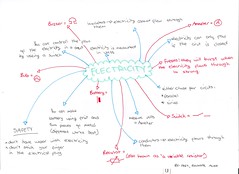
Here's an idea...
Brainstorm a topic without using books to find out what you already know and how you think the concepts link together. Then using another colour add to your brainstorm by using books and notes. You have a instant picture of the topic and an idea of what you know already (low priority revision) and what you don't (high priority revision). Here are some that 7R did today.
Electricity.
Matter.
Growing up.
Forces.
Tuesday, May 31, 2005
A challenge question for all years!
One minute, an organism is alive. The next minute, it is dead. What is the difference? Comments please!
Monday, May 30, 2005
Friday, May 27, 2005
MYTHICAL CREATURE DOMINOES
Never mind fun day this is where the real fun starts with the Mr Moore snack card challenge.
Use the Internet or your friendly local SD Resource Centre to work out the answer to this puzzle. The first correct answer wins a 50 Baht Snack Card.
If that first correct answer is also fully illustrated, it wins three 50 Baht Snack Cards!


Many mythical creatures are made up from parts of other creatures that really do exist. For the examples above, mermaids are a combination of fish and human; harpies are a combination of human and bird.
In the pictures above, you will see that, as in the game of Dominoes, it is possible to follow a trail from one creature to the next:
Fish/Human > Human/Bird > Bird/Lion
Below, but in alphabetical order only, are 9 different mythical creatures. Can you win the snack card challenge by placing them in a single line, so that each creature shares at least one component with the creature next to it?
CENTAUR
CHIMAERA
ECHIDNA
GRIFFIN
KINNAREE
MINOTAUR
PEGASUS
SATYR
SPHINX (Greek, not Egyptian)
Pass your answer to Mr Moore or if you have a question post it as a comment.
Use the Internet or your friendly local SD Resource Centre to work out the answer to this puzzle. The first correct answer wins a 50 Baht Snack Card.
If that first correct answer is also fully illustrated, it wins three 50 Baht Snack Cards!


Many mythical creatures are made up from parts of other creatures that really do exist. For the examples above, mermaids are a combination of fish and human; harpies are a combination of human and bird.
In the pictures above, you will see that, as in the game of Dominoes, it is possible to follow a trail from one creature to the next:
Below, but in alphabetical order only, are 9 different mythical creatures. Can you win the snack card challenge by placing them in a single line, so that each creature shares at least one component with the creature next to it?
CHIMAERA
ECHIDNA
GRIFFIN
KINNAREE
MINOTAUR
PEGASUS
SATYR
SPHINX (Greek, not Egyptian)
Pass your answer to Mr Moore or if you have a question post it as a comment.
Thursday, May 26, 2005
Guest speaker Professor Christoph Menke
 Today in the department Professor Christoph Menke (University of Applied Sciences, Trier, Germany and The Joint Graduate School of Energy and Environment, King Mongkut’s University of Technology, Thonburi) was invited to speak to year 12 Ecosystems and Physics students about the topic of 'Wind Energy – Issues and Options for One Renewable Energy Resource'. We thank him for taking time to share his work and for providing us with a power point presentation of his lecture (click here 25Mb *.ppt - available in school only). Comments from those who attended are welcome.
Today in the department Professor Christoph Menke (University of Applied Sciences, Trier, Germany and The Joint Graduate School of Energy and Environment, King Mongkut’s University of Technology, Thonburi) was invited to speak to year 12 Ecosystems and Physics students about the topic of 'Wind Energy – Issues and Options for One Renewable Energy Resource'. We thank him for taking time to share his work and for providing us with a power point presentation of his lecture (click here 25Mb *.ppt - available in school only). Comments from those who attended are welcome.
Friday, May 20, 2005
For all you bored Billy's out there over the holiday
 You know it is the simple games that are always the best. Use simple 'centre of mass' principles to balance bored Billy's books - Mr Roff has the record so far. What level can you get too?
You know it is the simple games that are always the best. Use simple 'centre of mass' principles to balance bored Billy's books - Mr Roff has the record so far. What level can you get too?
Thursday, May 19, 2005
How much fizz?
 Chemistry Club investigates - which is the fizziest carbonated drink?
Chemistry Club investigates - which is the fizziest carbonated drink?Did you know that dropping sand into a carbonated drink releases the dissolved carbon dioxide as bubbles? After extensive testing (of four varieties of carbonated soft drink) "coke" was found to contain the most fizz.
Wednesday, May 18, 2005
7R's homework and a challenge for CSI wannabees

Can you solve the mystery as a forensic scientist?
Click here to play, but take some time to read the insructions by clicking the help button first. Good luck in your mission.
Tuesday, May 17, 2005
We like to burn things in Science
 The student's in 8Y really like to set things on fire. Which do you think contains more energy, sugar or artificial sweetener? They set fire to both and recorded the temperature rise for a known volume of water. There is something wrong with the apparatus on this photo though - can you spot it?
The student's in 8Y really like to set things on fire. Which do you think contains more energy, sugar or artificial sweetener? They set fire to both and recorded the temperature rise for a known volume of water. There is something wrong with the apparatus on this photo though - can you spot it?
Saturday, May 14, 2005
Game for the weekend
 Sorry for not posting something for a while - been a little busy looking for tough questions for the year 7, 8 & 9 annual exams. Whilst searching I came across a few games that I would like to share with you. This week's game is called OMEGA SECTOR. You have to dock (land) your spaceship with a space station by controlling both the direction and size of the thrust. That's not all; you have to use the gravitational attraction of planets to bend the path of the spaceship whilst aliens try to shoot you. Try it and you'll see how addictive it is. I was defeated by level 11. Have fun. (you need fash installed)
Sorry for not posting something for a while - been a little busy looking for tough questions for the year 7, 8 & 9 annual exams. Whilst searching I came across a few games that I would like to share with you. This week's game is called OMEGA SECTOR. You have to dock (land) your spaceship with a space station by controlling both the direction and size of the thrust. That's not all; you have to use the gravitational attraction of planets to bend the path of the spaceship whilst aliens try to shoot you. Try it and you'll see how addictive it is. I was defeated by level 11. Have fun. (you need fash installed)
Monday, May 9, 2005
Science Competition for Thai Students
Are you interested in entering a competition for Biology, Physics or Chemistry? Click here for more details.
Friday, May 6, 2005
Year 10 Env Man River Trip
Monday, May 2, 2005
Win $1,000,000
 By answering all the questions on this States of Matter quiz you could become a millionaire. Good luck - remember to send me some of your winnings! Thanks to Mr Moaks and Booky for sending us the site.
By answering all the questions on this States of Matter quiz you could become a millionaire. Good luck - remember to send me some of your winnings! Thanks to Mr Moaks and Booky for sending us the site.
Subscribe to:
Posts (Atom)