 Jim Al-Khalili, theoretical physicist, University of Surrey
Jim Al-Khalili, theoretical physicist, University of SurreyI have given this some thought and it is a toss-up between two books.
The first is Dan Dennett's Consciousness Explained. As a physicist I enjoy reading about something I do not know much about. Dennett's book really opened up a whole new world for me. His rational, mechanistic and reductionist view of consciousness and how it manifests itself was a revelation. It is most likely not the whole answer, particularly in the light of research over the past decade or so in AI, cognitive studies and neuroscience. But I found it incredibly convincing and enlightening.
The second is Roger Penrose's Shadows of the mind. This was Penrose's first popular science book and sparked worldwide interest linking many ideas in quantum mechanics, the nature of time, artificial intelligence and the root of consciousness. I did not, and do not, agree with Penrose's main thesis on the quantum origins of consciousness in the brain. But there was so much more to this book, on non-computability, the meaning of quantum mechanics and its connection to other fields of physics that it profoundly affected my thinking.
If I had to choose, I would go for Dennett though.
Philip Ball, authorI'd like to suggest two candidates. The first is a widely recognized classic: D'Arcy Wentworth Thompson's On Growth and Form, first published in 1917 and then in revised and expanded form in 1942 (that version is available as a Dover reprint, 1992). Not all of the science in this book is accurate, and some is wrong. Much of it has been superseded, and in fact Thompson was writing well ahead of his time, before the tools and concepts were really developed to tackle many of the problems he considered. All the same, it is a great book. For one thing, it challenges the notion, still apparent today, that somehow biology escapes the constraints that apply to the rest of the physical world: Thompson was refuting the tendency in his time to explain all biological form with the black box of Darwinism. It shows the value of taking a synthetic view that cuts across scientific disciplines. In short, it provides nothing less than a new way of looking at the natural world. And it is written beautifully and with stunning erudition.
The second is barely known: Norbert Wiener's Invention (written in the 1950s but not published until 1994 by MIT Press). Wiener is best known for cybernetics, but here he makes an elegant case for the importance of the often overlooked creative strand of science evident in the process of invention. He explains how invention can be, and has been, nurtured, and what can go wrong in an intellectual climate to suppress it. To my mind, this book shows why the claim that science is somehow different from technology is not just wrong but pernicious.
Susan Blackmore, psychologist, writer and broadcasterI tried to think up something original or quirky but in the end I have to say my favourite is The Selfish Gene. It taught me, in beautiful language, the simplicity and power of Darwin's dangerous idea - "the best idea anybody ever had" and inspired all my subsequent work on memes.
David Deutsch, physicist, University of OxfordI'm afraid I find it impossible to choose between Gödel, Escher, Bach and The Selfish Gene. Both are examples of perfection in popular science writing: they deal with issues that are deep and important, but subtle enough that they are usually only discussed by specialists, and they make them accessible to the general reader through the authors' superlative writing skill and total command of their subjects.
K Eric Drexler, nanotechnology pioneer, researcher and authorMathematics, Form and Function by Saunders Mac Lane. Written by a pioneer of category theory, which links parallel structures across all areas of mathematics, this book provides a survey and integration of mathematics as a whole. It both shows the roots of mathematics in concrete human experience and explores the content of fields at the heights of abstraction, emphasizing their surprising connections. This is a book to be skimmed, then read, then revisited and studied.
Marcus du Sautoy, Professor of Mathematics, University of OxfordHardy's A Mathematician's Apology is a book I was recommended by teacher at school to read when I was 12 or 13. It was a revelation. It brought alive what mathematics is really about. It was like hearing real music for the first time after practising scales and arpeggios for years. I think everyone has a sense of what the chemist, physicist or biologist is investigating in their laboratories but giving someone access to the mathematician's lab is a much tougher job. Hardy shows that mathematics is as much a creative art as a useful science and that really appealed to someone who loved music as much as numbers. He gives two proofs in the book: they are simple but rapier-like in their logic. Being exposed to the power of this logical language to prove things with 100% certainty was very empowering to an adolescent whose world was constantly shifting.
As an adult it is a book I love and hate because it comes with a very mixed message. Anyone who wants to emulate Hardy and bring the subject alive for others lives under the spectre of the opening sentence of the book:
“It is a melancholy experience for a professional mathematician to find himself writing about mathematics. The function of a mathematician is to do something, to prove new theorems, to add to mathematics, and not to talk about what he or other mathematicians have done.”
I have spent my adult life trying to prove Hardy wrong: that it is possible to create new mathematics alongside providing access for others to this magical world.
Richard Fortey, senior paleontologist, Natural History MuseumI would like to nominate Oliver Sacks' book The Man who Mistook his Wife for a Hat. Sacks manages to explain much about how the human brain works, but in a way which is absolutely gripping as literature. He avoids the pitfalls of becoming too precious - everything is perfectly judged. He also has the prize for the most intriguing title - out Goulding Gould!
Baroness Susan Greenfield, Director of the Royal InstitutionHow to Build a Time Machine, by Paul Davies. Hugely original in terms of format. It looks easy – like a picture book – but introduces you to lots of difficult concepts.
Steve Jones, Professor of Genetics, University College LondonTo me one obvious winner - Charles Darwin's The Voyage of the Beagle. No other book so conveys the excitement of doing science, getting results and having a wonderful time, all at the same time. And, as well as a great science book, it's one of the best travel books ever written, with more adventures on a single page than most modern writers manage to squeeze into a chapter, or an entire book.
Marek Kohn, authorMason & Dixon, by Thomas Pynchon. ‘”The mists rise up out of the Bog. There she is, full, spherickal ... the last time I shall see her as a Material Being ... when next appearing, she will have resum'd her Deity.” Maskelyne will edit this out, which is why Mason leaves it in his Field Report.’ Thus Pynchon imagines the eighteenth-century astronomer Charles Mason observing a Transit of Venus: subtle phrasing, prose that sustains lasting pleasure, mischief, irony and wit; powerfully imparting a sense of science in a world still haunted and occult, and reminding the reader of all that is absent from conventional science writing.
Tim Lott, authorMy favourite science book is The Blank Slate by Stephen Pinker. It really opened my eyes to a huge number of ways in the way the general public and the media misperceive the nature of human nature, out of a misplaced need for control over the human personality. Pinker's remarkable book re-establishes inborn human nature as being at the heart of the personality, and does it without in any way handing the baton over to determinism, or ignoring the importance of environmental factors. I have read and re-read this book, and it has changed the way I think not only about the human mind, but how society allows it wishes to impinge on its ability to judge matters of science on the evidence available. A brave, groundbreaking victory for both common sense and free speech, The Blank Slate forces us to look at many of the deepest assumptions that our society holds and makes us start to radically revise them.
Mark Miodownik, lecturer in Mechanical Engineering, King’s College London submitted a list that included The Hitchhiker’s Guide to the Galaxy
My main problem, I have come to realise, is that I read The Selfish Gene and Day of the Triffids too early and so became both paranoid and solipsistic at far too tender an age. While other children were laughing heartily at Terry Pratchett's bonkers prose or smiling tenderly at A Wrinkle in Time, I was pondering the implications of A Brief History of Time and trying to understand why the publishers ever went ahead with it. When I finally got round to reading the The Hitchhiker's Guide to the Galaxy, I was a shocked and serious teenager, and I found the book so much funnier and cleverer than any other book I had read that I wore my dressing gown to school for a week, and would frequently burst into hysterical laughter at the mere mention of the word 'petunia'.
Toby Murcott, science writerMy book would be Surely You’re Joking, Mr Feynman. Richard Feynman gives the best insight into genius that I have ever read. He constantly baffles and amazes people by doing the obvious. His particular genius is to see the world with childlike eyes, ignore his beliefs and prejudices and just take problems at face value.
Vivienne Parry, writer and broadcasterMy favourite science book by a mile is The Voyage of the Beagle. It sits by my bed and I often dip into it. In fact I've just had to replace it for the 3rd time as it has been loved to death and fallen apart. It's a book written by an enthusiast who noted everything around him, plants, animals, rocks and people. It works as a terrific travel book, as a riveting insight into the scientific journey of one of the world's great scientists and as a great read. What more could you want?
Steven Pinker, Professor of Psychology, Havard UniversityThomas Schelling, The Strategy of Conflict, 1960. A masterpiece by a humane Dr. Strangelove, recent winner of the Nobel Prize in economics. This book introduced dozens of mind-blowing ideas on culture, emotion, conflict, cooperation, communication, and social life, whose full implications we are only beginning to explore. It is packed with wit and insight, and uses mathematical game theory judiciously. Malcolm Gladwell's The Tipping Point and Robert Frank's Passions within Reason, both excellent bestsellers, were based on Schelling's ideas.
Matt Ridley, authorThe Selfish Gene by Richard Dawkins. I still recall a sense of slight bewilderment when I read the newly published book as a first-term undergraduate at Oxford. Was this chap’s theory right or not? Until now my teachers had helpfully divided the world of science into right and wrong ideas. But here, I suddenly realised, I was going to have to make up my own mind. The handrails had gone. Dawkins’s sentences had such rhythm, his words had such precision and his thoughts had such order that his book was tasty literature as well as nourishing argument.
Dr Rowan Williams, Archbishop of CanterburyOliver Sacks, A Leg to Stand On, 1984. Challenges all sorts of assumptions about mind and body, and sketches a very exciting concept of the body itself as ‘taking shape’ in mind and imagination.
Heinz Wolff, former Head of the European Manned Space CommissionMy entry for the best science book ever is of course a very personal choice. The Microbe Hunters by Paul de Kruif, published first in 1926, did more to inspire me into science and medicine, than anything else I have read since. It has been republished quite frequently and can be bought readily in various formats from Amazon.
 1) Power from mains to antenna, which is made of copper
1) Power from mains to antenna, which is made of copper
 Hard to believe? The facts are easy to find. From published data, we can find that TNT yields about 4.25 megajoules of energy per kilogram when detonated, fairly typical for a high explosive.
Hard to believe? The facts are easy to find. From published data, we can find that TNT yields about 4.25 megajoules of energy per kilogram when detonated, fairly typical for a high explosive. No comments please! The year 12 biologists are out on their field trip at the moment (no wonder it is so quiet) and you may be wondering if the physicists go somewhere. Well, a couple of weekends back they went measuring, recording, calculating and experimenting (including measuring the g-force of each ride with a home made g-force device) at dreamworld. Mr Roff asked for them to send some interesting photos and this is what he they sent him. Who said physics was boring?
No comments please! The year 12 biologists are out on their field trip at the moment (no wonder it is so quiet) and you may be wondering if the physicists go somewhere. Well, a couple of weekends back they went measuring, recording, calculating and experimenting (including measuring the g-force of each ride with a home made g-force device) at dreamworld. Mr Roff asked for them to send some interesting photos and this is what he they sent him. Who said physics was boring?

 A device that repels teenagers has won the peace prize at this year's Ig Nobels - the spoof alternative to the rather more sober Nobel prizes. Welshman Howard Stapleton's device makes a high-pitched noise inaudible to adults but annoying to teenagers.
A device that repels teenagers has won the peace prize at this year's Ig Nobels - the spoof alternative to the rather more sober Nobel prizes. Welshman Howard Stapleton's device makes a high-pitched noise inaudible to adults but annoying to teenagers.


 You've never heard a sound effect quite like this coming out of an ordinary cup!
You've never heard a sound effect quite like this coming out of an ordinary cup!
 You will need:
You will need:
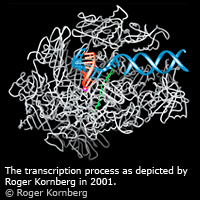 PCR is a method by which a few fragments of DNA can be duplicated into millions in a couple of hours. This makes PCR a very useful method in forensic science, as it means that very small amounts of DNA could be enough to identify a person. PCR was invented by Kary Mullis, one of two Nobel Laureates in Chemistry in 1993. If you play the game below, you will be able to learn more about PCR. Also, a must for HL Biology and Chemistry students is the 30 min lecture by Roger D. Kornberg, the winner of the 2006 Nobel Prize for Chemistry for his studies of the molecular basis of eukaryotic transcription.
PCR is a method by which a few fragments of DNA can be duplicated into millions in a couple of hours. This makes PCR a very useful method in forensic science, as it means that very small amounts of DNA could be enough to identify a person. PCR was invented by Kary Mullis, one of two Nobel Laureates in Chemistry in 1993. If you play the game below, you will be able to learn more about PCR. Also, a must for HL Biology and Chemistry students is the 30 min lecture by Roger D. Kornberg, the winner of the 2006 Nobel Prize for Chemistry for his studies of the molecular basis of eukaryotic transcription. Finished your drink of juice? Great! No don’t throw away the empty carton! Here’s something fascinating….
Finished your drink of juice? Great! No don’t throw away the empty carton! Here’s something fascinating….

 You will need:
You will need: Imagine the air in a small soap bubble, about the size of the tip of your little finger. All those little molecules dashing about bumping into each other.
Imagine the air in a small soap bubble, about the size of the tip of your little finger. All those little molecules dashing about bumping into each other.
 Caution: this rocket can fly up to 3-4m. Adult supervision required.
Caution: this rocket can fly up to 3-4m. Adult supervision required.
 What you need:
What you need: The Centre for Bioscience at The Higher Education Academy, University of Leeds, are launching a photographic competition on the theme of 'Bioscience in Action' where they are asking for entries from non-professional photographers on any angle of bioscience, including teaching it. The prizes are £500, £200 and £100 for first, second and third winners. There’s a few rules and regs, those and the entry forms etc. are all on
The Centre for Bioscience at The Higher Education Academy, University of Leeds, are launching a photographic competition on the theme of 'Bioscience in Action' where they are asking for entries from non-professional photographers on any angle of bioscience, including teaching it. The prizes are £500, £200 and £100 for first, second and third winners. There’s a few rules and regs, those and the entry forms etc. are all on 

 Hints:
Hints: A German scientist has used nanotechnology to create what he believes is the world's smallest soccer pitch.
A German scientist has used nanotechnology to create what he believes is the world's smallest soccer pitch.
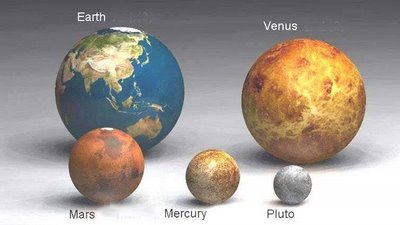
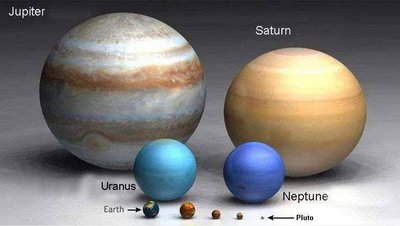
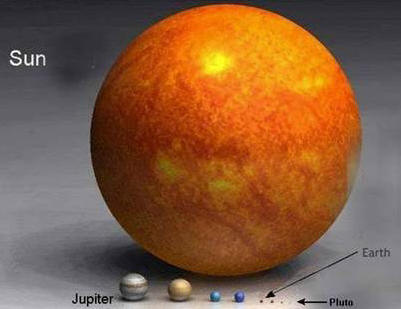

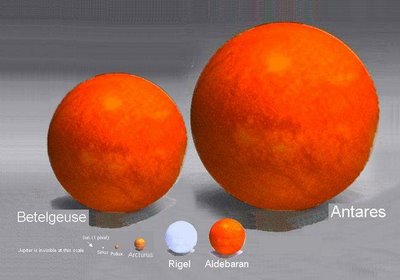
 When is a planet not a planet? That is the question. At least according to the International Astronomical Union (IAU). For the first time, the organisation will be officially defining the word "planet", and it is causing much debate in the world of astronomy. There is only one thing that everyone seems to agree on: there are no longer nine planets in the Solar System.
When is a planet not a planet? That is the question. At least according to the International Astronomical Union (IAU). For the first time, the organisation will be officially defining the word "planet", and it is causing much debate in the world of astronomy. There is only one thing that everyone seems to agree on: there are no longer nine planets in the Solar System.

